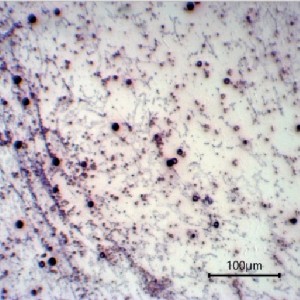
Cytomorphological analysis of liquid platelet-rich fibrin produced with the DUO fixed angle centrifuge (Process, France) for use in the regenerative therapy of skin ulcers
Submitted: June 3, 2023
Accepted: October 16, 2023
Published: December 14, 2023
Accepted: October 16, 2023
Abstract Views: 159
PDF: 43
Supplementary Materials: 14
PDF (Italiano): 68
Materiali Supplementari (Italiano): 18
Supplementary Materials: 14
PDF (Italiano): 68
Materiali Supplementari (Italiano): 18
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Angela Peghetti, Diletta Olivari, Silvia Tedesco, Andrea Bellingeri, Marino Ciliberti, Antonio Di Lonardo, Corrado M. Durante, Antonino Grasso, Elia Ricci, Alessandro Scalise, Giovanni Pomponio, Prontosan®solution and Prontosan®Debridement Pad in the treatment of different types of cutaneous wounds: expert-based statements, case series and review of the literature , Italian Journal of Wound Care: Vol. 3 No. 2 (2019)
- Giovanni Petracca, Francesco Zappia, Giuseppe Maccarone , Necrotizing fasciitis: A case report and a review of the literature , Italian Journal of Wound Care: Vol. 5 No. 2 (2021)
- Elsa Vitale, Lucia Rosa De Angelis, Francesco Germini, Techniques and solutions used in cleansing ulcers: a review of the literature , Italian Journal of Wound Care: Vol. 4 No. 1 (2020)
- Andrea Pazzini, Barbara Biselli, Chiara Vannini, Elisabetta Fabbri, Felice Falabella, Maria Giulia Santandrea, Marianna Marziliano, Nicole Gagliardi, Sara Di Giandomenico, Simona Scotto di Minico, Vito Di Biasi, Can the Braden scale predict the development of pressure injuries in intensive care? , Italian Journal of Wound Care: Vol. 7 No. 3 (2023)
- Francesco Petrella, Giuseppe Nebbioso, Marcello Aquino, Mario Bellisi, Sara Carella, Rosaria Grace Catalfamo, Diletta Donateo, Pasquale Esposito, Pierluigi Gallo, Maria Grazia Mezzasalma, Stefano Riccardi, Concetta Romano, Alessandro Scanguzzi, Observational study of a gauze medication contaning Rigenase® and polyhexanide in the treatiment of venous leg ulcers (<6cm2) and perilesional skin , Italian Journal of Wound Care: Vol. 6 No. 2 (2022)
- Deborah Cesura Granara , Anna Baxa, Cinzia Viaggi, Cristina Pruzzo, Giorgia Piana, Giorgio Badino, Miriam Roccaforte, Silvia Pienovi, Tiziana Zuliani, Marco Marchelli, Giuseppe Perniciaro, Carmelo Gagliano, Luigi Carlo Bottaro, Impact of the wound hygiene concept on wound contraction: an experience by ‘Vulnologia ASL3 Regione Liguria’ , Italian Journal of Wound Care: Vol. 7 No. 3 (2023)
- Elia Ricci, Monica Pittarello, Use of the Lumiheal device in the treatment of contaminated wounds , Italian Journal of Wound Care: Vol. 8 No. 1 (2024)
You may also start an advanced similarity search for this article.



 https://doi.org/10.4081/ijwc.2023.103
https://doi.org/10.4081/ijwc.2023.103






